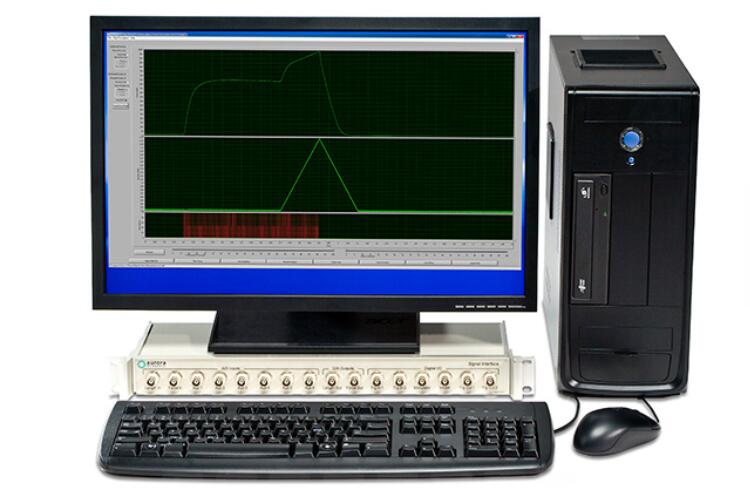
1305A: 3-in-1 Whole Animal System – Rat
型号:1305A
价格:请致电:010-67529703
品牌:aurorascientific

Overview
The 3-in-1 Whole Animal System delivers accurate and flexible measurement of rodent muscle properties in-situ, in-vivo and in-vitro. Ideal for live animal protocols, including injury and longitudinal studies, it evaluates the mechanical characteristics of intact muscle of rats, either isolated in-situ, with a footplate (ex-vivo) or isolated in-vitro. Experimental setup, data collection and data analysis can all be done in a matter of minutes.
By combining in-situ, in-vivo and in-vitro muscle tests, researchers are able to capture a complete picture of muscle physiology in rats. The test system comes with the rat apparatus, complete with temperature controlled animal and limb plates designed to support and fix the animal and limb being testing. It can be converted to an isolated (in-vitro) muscle test system with the attachment of an optional 25 mL bath. Aurora Scientific’s flagship Dual-Mode lever system is also included, permitting measurement and control of both force and length.
Muscle samples are attached at only one point to measure force and length saving time and increasing productivity. The system includes our high-power, field stimulator and all required electrodes.
Control and analysis software comes pre-loaded on a custom PC. Experimental setup, data collection and data analysis can all be done in a matter of minutes with our control and analysis software (DMC/DMA). Parameters such as resting length, resting force, stimulation and the actual test protocol are all set using the control software. An extensive library of standard experimental protocols such as twitch, tetanus, fatigue, force-frequency, force-velocity, stiffness and work loops are also provided with the system.

Tell the Whole Story – Live Animal & Isolated Tissue in One
The three system configurations allows the researcher to work with a broad range of muscle types, providing a convenient platform for compound screening, phenotype evaluation and comparison of murine models; all within one system.
High Throughput Software Capability
Experience high-throughput data analysis, including batch processing and multi-parameter calculations for hundreds of muscle samples within minutes. Downstream analysis can be completed within Aurora Scientific DMC/DMA software or exported to your analysis program of choice.
Standard Protocol Library
The protocol library includes a variety of muscle experiments for rat in vitro studies. Protocols include system operation and data acquisition settings optimized for sample type and measurement needs. Add your own custom protocols as well to streamline system operation with multiple lab members.
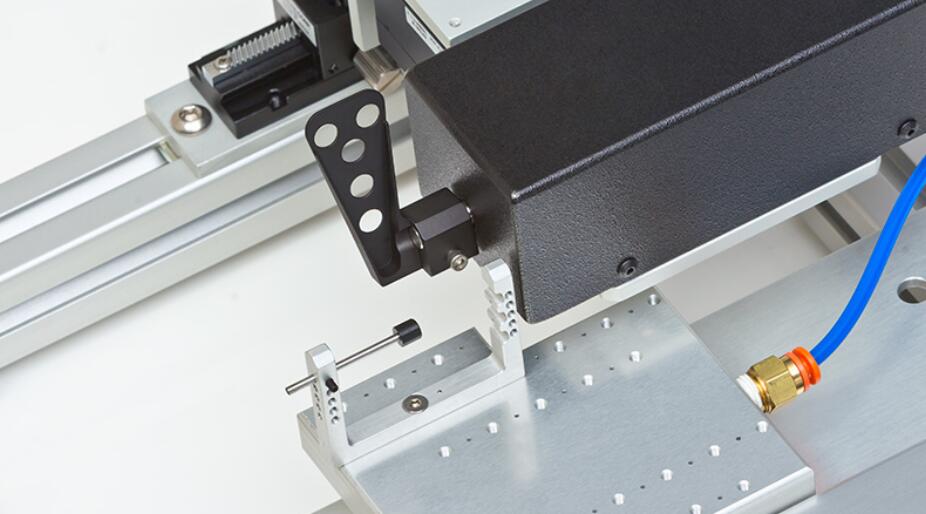
Features
Case Studies
In 2012, Marius Locke at the University of Toronto had been studying letal muscle damage and the role that heat shock proteins (HSPs) play in protecting the muscle from stress induced damage, such as exercise. Dr. Locke was looking for equipment that could reliably and repeatedly induce muscle damage in his rats while accurately quantifying force production and recovery.
Our 1305A system was suggested for the job as it had the ability to perform the kinds of repeated contractions that Dr. Locke was looking to accomplish. It was first implemented piecemeal to integrate with some existing equipment in the laboratory, but because of its ease of use, the remaining Aurora Scientific pieces were added for a complete system. Aurora Scientific technical support helped craft the best protocols for Dr. Locke’s experiments and soon thereafter the first cohort of rats was tested.
The system has helped Dr. Locke and his graduate students publish valuable findings about the protective role of HSPs in damaged muscle. In addition, Dr. Locke established an important rat model of standard human exercise protocols to gain valuable, physiologically relevant data which may one day be applicable to athletes and people alike.
Citations
Rooney, Jachinta and Rich Lovering. “Single muscle contractile measurements in vivo and in situ.” NIHSOP: MDC1A_M.2.2.002 (2015): 1-8.
Holwerda, Andrew M. and Marius Locke. “Hsp25 and Hsp72 content in rat letal muscle following controlled shortening and lengthening contractions.” Applied Physiology, Nutrition and Metabolism 39.12 (2014): 1380-1387.
DeRuisseau, Keith C. et al. “Aging-related changes in the iron status of letal muscle.” Experimental Gerontology 48.11 (2013): 1294-1302.
Ibebunjo, Chikwendu et al. “Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia.” Molecular and Cellular Biology 33.2 (2013): 194-212.
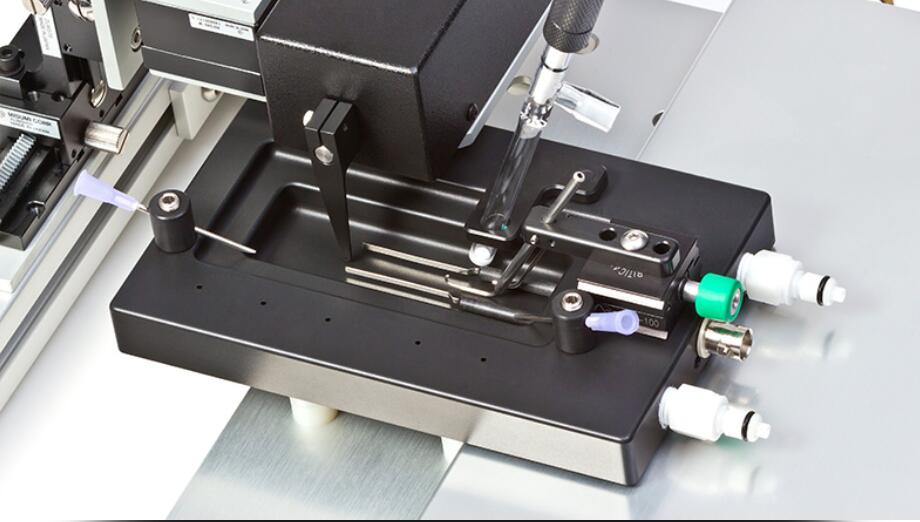
1305A – Footplate Configuration for Rats Invaluable to the Study of Muscle Damage
CHALLENGE
SOLUTION

RESULTS